Small is Powerful: A Globally Unique Capability for Nanoforming HPAPIs
By Dr. David Rowe – Head of Manufacturing and Antonio da Silva – Senior Director Business Operations, Nanoform
In oncology and the wider pharmaceutical market, there is a surge in demand for high- potency active pharmaceutical ingredients (HPAPIs). These are actives at lower doses, resulting in fewer side effects for patients and requiring lower amounts of API to be produced (1,2). Reducing the particle size of HPAPIs down to the nanoscale also provides the opportunity for lower dosage. It can provide additional benefits, such as necessitating fewer excipients, faster action, and enhancing bioavailability, a leading cause of clinical attrition. (3).
However, their potency makes HPAPIs challenging to handle safely, necessitating specialized facilities. Nanoform is unique as a nanoparticle medicine enabling company with small-molecule and biological nanoparticle technologies with investments in HPAPI handling capabilities. Its facilities can handle small-molecule HPAPI particles as small as 10 nm, with an occupational exposure limit (OEL) of 30 ng/m3 (referring to the upper limit on the acceptable concentration of an HPAPI). This ambitious expansion project arose due to client demand and Nanoform’s commitment to support the development and manufacture of HPAPI nanoparticles and to enable these promising drug substances to progress safely.
Safety first
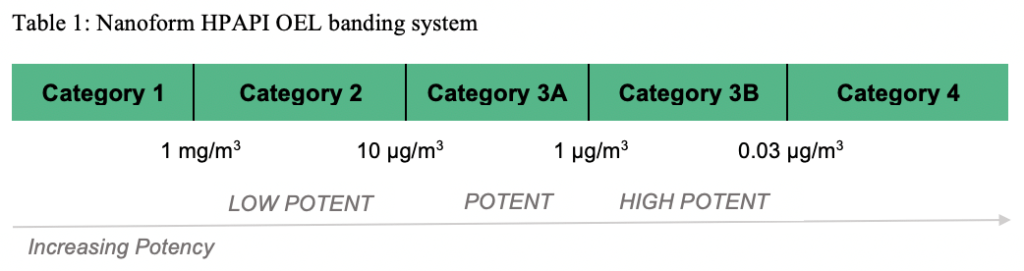
In the handling of all drugs and specifically HPAPIs, safety is paramount, followed immediately by quality. Exposure to HPAPIs must be kept at acceptable levels for individuals working with the material while consistency in batch production is necessary to mitigate risk to patients. Nanoform previously was able to handle HPAPIs up to 1 μg/m3, falling within the SafeBridge Category 3A (see table below), and Nanoform has recently invested in a GMP manufacturing facility expansion, adding two additional manufacturing suites, together with GMP analytical characterization laboratories, which can handle highly potent compounds to less than 30 ng/m3 (Cat 3B).
The journey of a HPAPI at Nanoform starts with an onboarding questionnaire, followed by an internal safety evaluation before any project is agreed to progress and any material is received on site. Following this and in consultation with the API owner, the OEL is determined, and the correct containment procedure for handling the HPAPI is put in place. Nanoform’s proprietary CESS® nanoforming process can then take place, in which the dry-powder API is dissolved in supercritical carbon dioxide and recrystallized under controlled temperature and pressure to form nanoparticles that are tunable in size, shape and polymorphic form. Unlike particle-reduction techniques such as spray-drying, CESS® can take place entirely inside an isolator which significantly reduces risk of exposure to the API. Additionally, during cleaning, in situ dissolution in supercritical carbon dioxide aids the cleaning process of the equipment to further reduce the risk of exposure.
Engineering controls consisting of closed systems, with isolators equipped with HEPA filters and other containment procedures built by design, are in place to achieve the necessary containment levels. Equipment is certified with Standardized Measurement for Equipment Particulate Airborne Concentrations (SMEPAC) testing from SafeBridge, a premier resource for high-level safety and health consulting to the pharmaceutical and biotechnology industry. The SMEPAC test is a standardized methodology for testing the effectiveness of systems and equipment for retaining dust particles, which:
- Demonstrates that the barrier technology reduces the presence of potentially dangerous particles in the air during working processes
- Checks the containment ability of systems
- Certifies that the manufacturers’ containment equipment is operating within set containment limits
Quality by design (QbD) is further integrated across all processes to ensure consistency. With these checks in place, the Nanoform team has the expertise and globally unique capabilities to take partners from initial safety evaluation through to preclinical formulations of nanoparticle HPAPIs and into clinic and beyond.
Maximizing success by combining game-changing AI with powerful capabilities
The high potency and low quantities needed for HPAPIs mean that they are a precious material, and batch failure can have significant financial consequences. Nanoform is equipped with the technologies and expertise to handle low amounts of HPAPI effectively while the high consistency and reliability of the CESS® process and integration of QbD mitigates the risk of batch failure.
In order to reduce time, cost and safety risks associated with experiments in the lab still further, Nanoform has developed a bespoke sparse-data AI engine: STARMAP® Online. This acts as a digital twin of CESS®, identifying the winning drug candidates before even opening the lab doors. As a preliminary step that takes place virtually, STARMAP® Online can streamline drug development and help pharmaceutical manufacturers invest financial resources in drugs with the highest probability of success.
A safe harbor for HPAPI particles
The enormous potential of HPAPI particles for patient-optimized therapeutics has led to a surge in interest around their development and a need for novel technology companies with the resources to handle these materials safely.
Would you like to learn how Nanoform’s experienced team can support your HPAPI development projects? Reach out to commercial@nanoform.com.
Citations
- https://www.marketdataforecast.com/market-reports/high-potency-active-pharmaceutical-ingredients-market
- https://www.ihealthcareanalyst.com/global-highly-potent-active-pharmaceutical-ingredients-market/
- Kalepu S, Nekkanti V. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharm Sin B. 2015 Sep;5(5):442-53. doi: 10.1016/j.apsb.2015.07.003. Epub 2015 Aug 24. PMID: 26579474; PMCID: PMC4629443.


