Nanomilling ― For Improved Solubility and Bioavailability
A crucial feature of drug development is bioavailability, defined as “the ability of a drug to be absorbed and used by the body.” For a drug to be bioavailable, it must first be soluble, meaning able to be dissolved, especially in water. Many drugs on the market today are poorly water soluble, and patent extensions or 505(b)(2) new patents become possible for formulation improvements delivered via nanomilling.
Nanomilling is a proven technique that can overcome solubility challenges, reduce risk of failure, and ensure your molecules are developed successfully into life-saving therapies.
Solubility Classifications
The Biopharmaceutics Classification System (BCS) is a guide provided by the US FDA for predicting a drug’s intestinal absorption. This system develops a prediction using the parameters of solubility and intestinal permeability (see Figure 1).
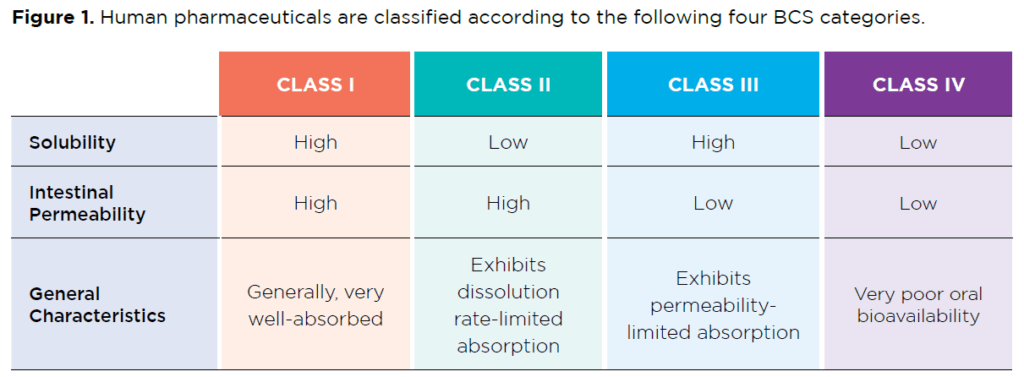
Forty percent of marketed drugs and 90% of active pharmaceutical ingredients (APIs) are poorly water-soluble, BCS Class II or IV. APIs that fall into BCS Class II are optimal candidates for particle-size reduction as their dissolution is the rate-determining factor in drug absorption.
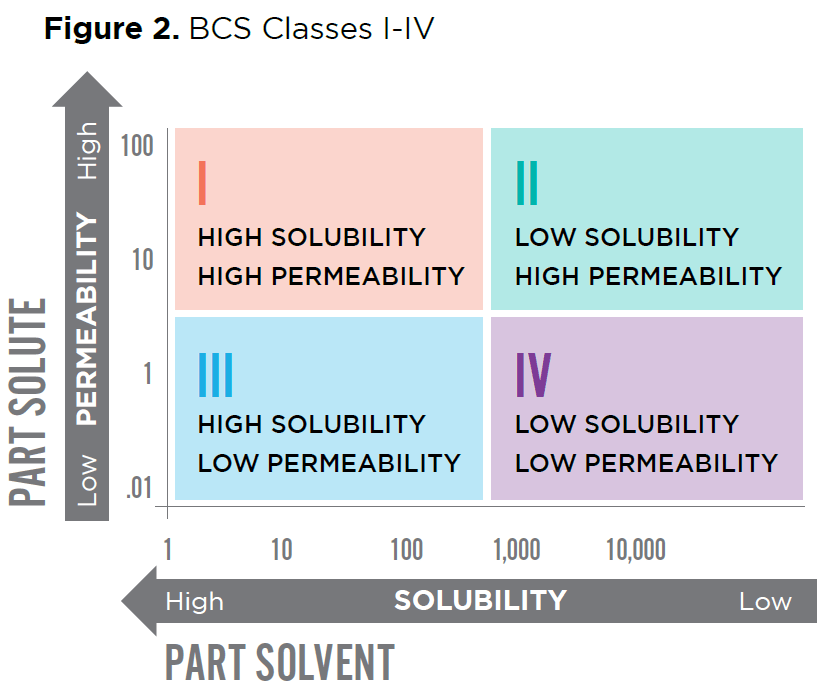
The BCS classes are depicted in Figure 2.
OVERCOMING SOLUBILITY CHALLENGES
Promising therapeutic molecules, even if classified as poorly soluble, can be developed successfully. There are multiple technologies available to increase the solubility and oral bioavailability of poorly soluble molecules being developed as APIs by reducing their particle size.
Nanomilling is a universal technique that can be applied to almost any API with water solubility below 200 μg/mL. It is a very adaptable drug-delivery platform suitable for oral, injectable, inhalable, and buccal applications, for which fine drug particulates are especially desired in formulations.
Nanomilling is a unit operation where mechanical energy is applied to physically break down coarse particles to finer ones. It has wide commercial and industrial applications as every drug can be ground to finer particles, whether aqueous or non-aqueous soluble. Decreasing the size of the API molecule increases the size of the specific surface area; the larger surface area allows for greater contact with water, increasing the API’s dissolution rate and bioavailability.
NANOMILLING BENEFITS
Benefits of particle-size reduction for the parenteral route include small dose volumes (resulting from high drug loading) and avoidance of harsh solvents and/or extreme pH conditions. Advantages for the pulmonary route include the ability to use inhalers intended for solutions as well as the ability to produce spray-dried powders whose particle sizes are optimized for deep lung delivery.
Other advantages include reduced fed/fasted variability in both liquid and solid dosage forms, faster onset of therapeutic action, low excipient side effects, and the ability to run continuously.
The process and equipment are easy to scale. Once the formulation and the process are optimized, very little batch-to-batch variation is observed in the quality of the dispersion.
The closed system allows for control of the milling environment as well as protection of the operators from exposure to potent drugs.
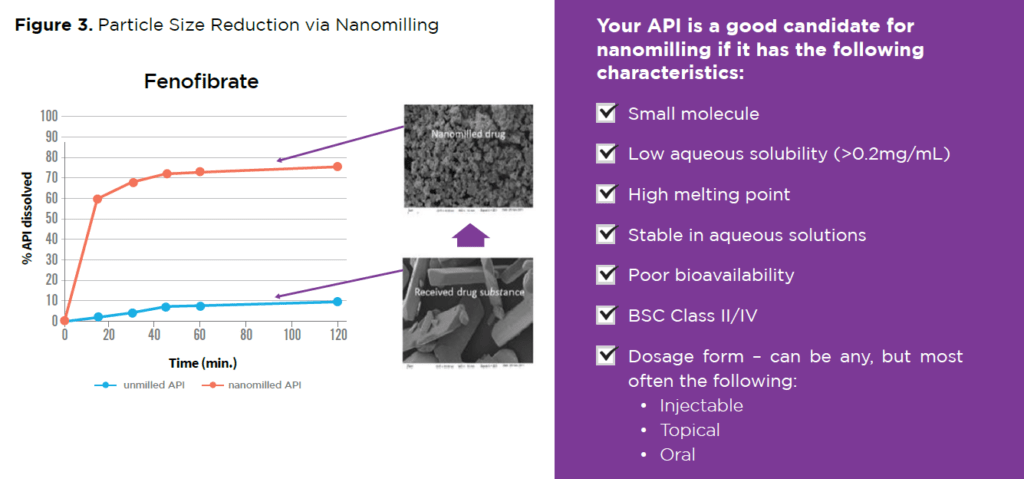
A real-world example of the significant change in fenofibrate particle size achieved via nanomilling is demonstrated in Figure 3.
EXPERIENCE YOU CAN TRUST
Nanomilling is a highly complex process requiring a unique level of CDMO expertise that can only be gained through extensive experience with developing a broad range of APIs.
The experts at Altasciences can take your API from formulation to commercialization. We have the necessary procedures, equipment, and experience to work with any formulation. Our highly skilled teams work with the latest equipment, including the Netzsch DeltaVita 15-300 mill, which can reduce particles to nanometer size with our wet milling options, fill vials in a range of sizes (from 0.3 mL to 500 mL), and package them.
For any question, or to partner for your drug development program, speak to our scientific experts.
ABOUT ALTASCIENCES
Altasciences is an integrated drug-development solution company offering pharmaceutical and biotechnology companies a proven, flexible approach to preclinical and clinical pharmacology studies, including formulation, manufacturing, and analytical services. For over 25 years, Altasciences has been partnering with sponsors to help support educated, faster, and more complete early drug-development decisions, helping them get better drugs to the people who need them, faster. Visit our website to discover our full range of drug-development and manufacturing solutions: https://www.altasciences.com/manufacturing-and-analytical-services
REFERENCES
- F. Thomas, “Looking Beyond the Solubility Horizon,” Pharmaceutical Technology 43 (6) 24–26 (2019).
- M. Li et al, “Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective,” Pharmaceutics 8 (2) 17 (2016); doi:10.3390/pharmaceutics8020017
- L. Peltonen et al., “Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods,” Journal of Pharmacy and Pharmacology, 62 (11) 1569–1579 (2010).
- Y. Golfar et al., “Prediction of Biopharmaceutical Drug Disposition Classification System (BDDCS) by Structural Parameters,” J Pharm Pharm Sci (www.cspsCanada.org) 22 (1) 247–269 (2019).



