New Drug Approvals in 2021: The Numbers and Trends
FDA approval of new molecular entities is a measure of product innovation in the bio/pharma industry. How did the industry fare in 2021? A look at the numbers and key trends.
By Patricia Van Arnum, Editorial Director, and Miranda Greenberg, Editorial Coordinator, DCAT
New drug approvals in 2021
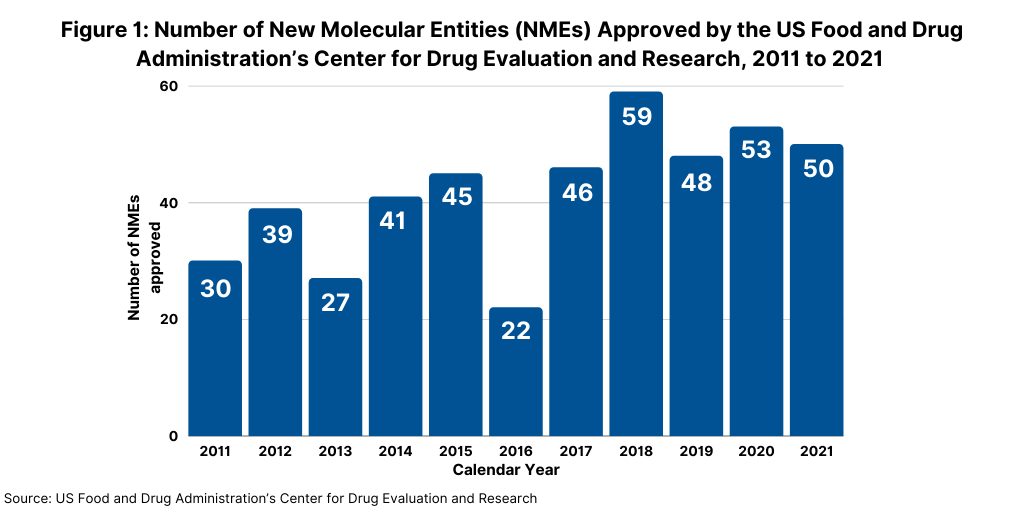
In 2021, the US Food and Drug Administration’s Center for Drug Evaluation and Research (CDER) approved 50 new molecular entities (NMEs), which was on par with the number of NMEs approved in 2020 and which continued a strong trajectory for new drug approvals. In 2020, FDA’s CDER approved 53 NMES, which was the second highest level of NMEs approved in a given year in the past decade, except for 2018 when 59 NMEs were approved (see Figure 1).
NME approvals: small molecules versus biologics
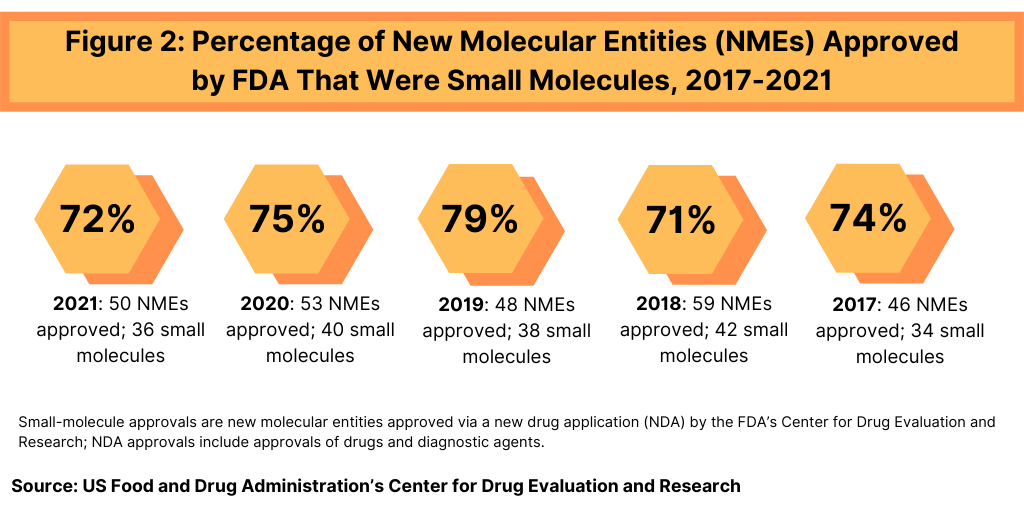
In terms of product mix, small-molecule drugs dominated NME approvals in 2021. Of the 50 NMEs approved in 2021, 36, or 72%, were small molecules, and 14, or 28%, were biologics. This continues a recent trend of having approximately three-fourths of NME approvals be small molecules (see Figure 2). In 2020, 75% or 40 of the 53 NMEs were small molecules. In 2019, 79% of NME approvals were small molecules; in 2018, it was 71% and 74% in 2017. Table 1 at end of article identifies small molecules approved in 2021 (approved as new drug applications) and biologics (approved as biologics license applications).
NME approvals: orphan drugs
In 2021, 26 of CDER’s 50 new drug approvals (52%) were approved to treat rare or “orphan” diseases (diseases that affect than 200,000 people in the US). This continues a recent trend in which approximately 40% to roughly 50% of NME approvals were for orphan drugs (see Figure 3). In 2020, 58%, or 31, of the 53 NME approvals in 2020 were orphan drugs. The 58% of NME approvals in 2020 matched a recent high in the share of orphan drugs in NME approvals; in 2018, 58%, or 34, of the 59 NMEs approved were orphan drugs (see Figure 3).
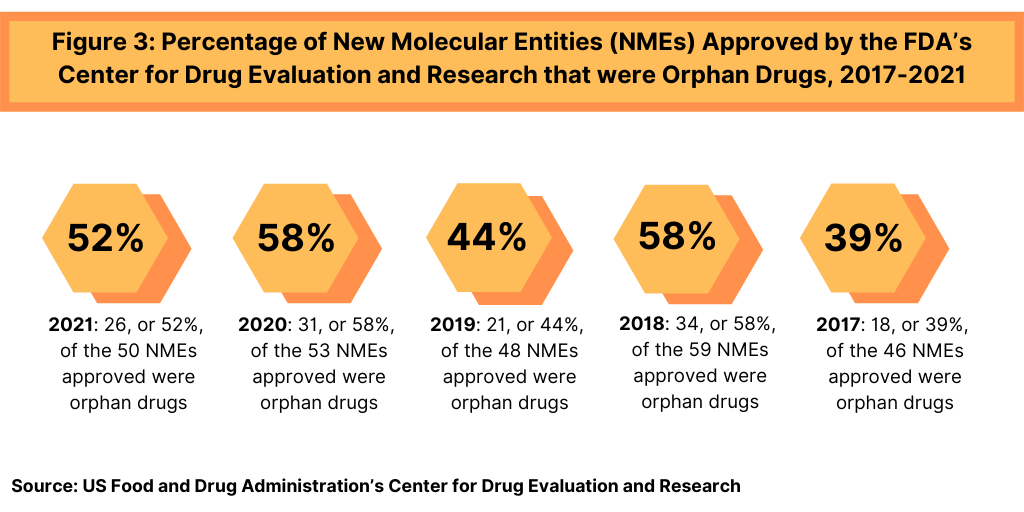
Table I (see below) identifies all 50 NME approvals in 2021.
Table I: New Molecular Entities Approved in 2021 by the FDA’s Center for Drug Evaluation and Research
| Company | Proprietary Name (active ingredient); NDA or BLA | Indication |
| AbbVie | Qulipta (atogepant); NDA | Episodic migraines |
| ADC Therapeutics | Zynlonta (loncastuximab tesirine-lpyl); BLA | Certain types of relapsed or refractory large B-cell lymphoma |
| Albireo | Bylvay (odevixibat); NDA | Pruritus |
| Alkermes | Lybalvi (olanzapine and samidorphan); NDA | Schizophrenia in adults and certain aspects of bipolar I disorder in adults |
| Amgen | Lumakras (sotorasib); NDA | Non-small cell lung cancer with the KRAS G12C genetic mutation called KRAS G12C |
| Apellis Pharmaceuticals | Empaveli (pegcetacoplan); NDA | Paroxysmal nocturnal hemoglobinuria, a rare blood disorder |
| Argenx | Vyvgart (efgartigimod alfa-fcab); BLA | Generalized myasthenia gravis |
| Ascendis Pharma Endocrinology Division A/S | Skytrofa (lonapegsomatropin-tcgd); BLA | Short stature due to inadequate secretion of endogenous growth hormone |
| AstraZeneca | Saphnelo (anifrolumab-fnia); BLA | Moderate-to severe systemic lupus erythematousus along with standard therapy |
| AstraZeneca | Tezspire (tezepelumab-ekko); BLA | Severe asthma as an add-on maintenance therapy |
| Aurinia Pharmaceuticals | Lupkynis (voclosporin); NDA | Lupus nephritis |
| Aveo Oncology | Fotivda (tivozanib); NDA | Relapsed or refractory advanced renal cell carcinoma |
| Bayer | Kerendia (finerenone); NDA | Reduce the risk of kidney and heart complications in chronic kidney disease associated with Type 2 diabetes |
| Biogen/Eisai | Aduhelm (aducanumab); BLA | Alzheimer’s disease |
| BioMarin Pharmaceutical | Voxzogo (vosoritide); NDA | Improve growth in children five years of age and older with achondroplasia and open epiphyses |
| Cara Therapeutics | Korsuva (difelikefalin); NDA | Moderate-to-severe pruritus associated with chronic kidney disease in certain populations |
| ChemoCentryx | Tavneos (avacopan); NDA | Severe active anti-neutrophil cytoplasmic autoantibody-associated vasculitis in combination with standard therapy, including glucocorticoids |
| Commave Therapeutics | Azstarys (serdexmethylphenidate and dexmethylphenidate); NDA | Attention deficit hyperactivity disorder |
| G1 Therapeutics | Cosela (trilaciclib); NDA | Chemotherapy-induced myelosuppression in adult patients with small-cell lung cancer |
| GlaxoSmithKline | Jemperli (dostarlimab-gxly); BLA | Recurrent or advanced mismatch repair deficient (dMMR) endometrial cancer as well as solid tumors, as determined by an FDA-approved test |
| Jazz Pharmaceuticals | Rylaze (asparaginase erwinia chrysanthemi [recombinant]-rywn); BLA | Acute lymphoblastic leukemia and lymphoblastic lymphoma in patients who are allergic to E. coli-derived asparaginase products, as a component of a chemotherapy regimen |
| Johnson & Johnson | Rybrevant (amivantamab-vmjw); BLA | Non-small-cell lung cancer with epidermal growth factor receptorexon 20 insertion mutations |
| Johnson & Johnson | Ponvory (ponesimod); NDA | Relapsing forms of multiple sclerosis |
| LEO Pharma | Adbry (tralokinumab-ldrm); BLA | Moderate-to-severe atopic dermatitis |
| Mayne Pharma | Nextstellis (drospirenone and estetrol); NDA | Contraceptive |
| Merck & Co. | Welireg (belzutifan); NDA | Von Hippel-Lindau disease under certain conditions |
| Merck & Co./Bayer | Verquvo (vericiguat); NDA | Chronic heart failure |
| Merck KGaA’s EMD Serono | Tepmetko (tepotinib); NDA | Metastatic non-small-cell lung cancer) with mesenchymalepithelial transition exon 14 skipping alterations |
| Mirum Pharmaceuticals | Livmarli (maralixibat); NDA | Cholestatic pruritus associated with Alagille syndrome |
| Novartis | Scemblix (asciminib); NDA | Philadelphia chromosome-positive chronic myeloid leukemia with disease that meets certain criteria |
| Novartis | Leqvio (inclisiran); NDA | Heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease as an add-on therapy |
| On Target Laboratories | Cytalux (pafolacianine); NDA | Help identify ovarian cancer lesions |
| Oncopeptides | Pepaxto (melphalan flufenamide); NDA | Relapsed or refractory multiple myeloma |
| Origin Biosciences | Nulibry (fosdenopterin); NDA | To reduce the risk of death caused by Molybdenum Cofactor Deficiency Type A, a rare, genetic metabolic disorder that typically presents in the first few days of life, causing intractable seizures, brain injury and death. |
| PharmaEssentia | Besremi (ropeginterferon alfa-2b-njft); BLA | Polycythemia vera |
| Progenics Pharmaceuticals | Pylarify (piflufolastat F-18 injection); NDA | Positron emission tomography imaging of prostate-specific membrane antigen-positive lesions in men with prostate cancer |
| QED Therapeutics/Helsinn | Truseltiq (infigratinib); NDA | Cholangiocarcinoma (bile duct cancer) under certain disease criteria |
| Regeneron Pharmaceuticals | Evkeeza (evinacumab-dgnb); BLA | Homozygous familial hypercholesterolemia, a rare condition marked by elevated circulating levels of low-density lipoprotein cholesterol (LDL-C) |
| Sanofi | Fexinidazole (fexinidazole); NDA | Human African trypanosomiasis caused by the parasite Trypanosoma brucei gambiense |
| Sanofi | Nexviazyme (avalgluclosidase alfa-ngpt); BLA | Late-onset Pompe disease |
| Sanofi* | Rezurock (belumossudil); NDA | Chronic graft-versus-host disease after failure of at least two prior lines of systemic therapy |
| Sarepta Therapeutics | Amondys 45 (casimersen); NDA | Duchenne muscular dystrophy with a confirmed mutation amenable to exon 45 skipping, a rare genetic disorder characterized by progressive muscle deterioration and weakness |
| Scynexis | Brexafemme (ibrexafungerp); NDA | Vulvovaginal candidiasis |
| Seagen (formerly Seattle Genetics) | Tivdak (tisotumab vedotin-tftv); BLA | Recurrent or metastatic cervical cancer with disease progression on or after chemotherapy |
| Supernus Pharmaceuticals | Qelbree (viloxazine); NDA | Attention deficit hyperactivity disorder in patients 6 to 17 years of age |
| Takeda | Exkivity (mobocertinib); NDA | Locally advanced or metastatic non-small cell lung cancer with epidermal growth factor receptor exon 20 insertion mutations |
| Takeda | Livtencity (maribavir); NDA | Post-transplant cytomegalovirus (CMV) infection/disease that does not respond to available antiviral treatment for CMV |
| TG Therapeutics | Ukoniq (umbralisib); NDA | Marginal zone lymphoma and follicular lymphoma |
| Viiv Healthcare | Cabenuva (cabotegravir and rilpivirine); NDA | HIV |
| Zealand Pharma | Zegalogue (dasiglucagon); NDA | Severe hypoglycemia |
FDA is US Food and Drug Administration
NDA is new drug application; BLA is biologics license application.
ADC Therapeutics’ Zynlonta (loncastuximab tesirine-lpyl) is an antibody drug conjugate.
Apellis and Sobi entered a collaboration to develop and commercialize systemic pegcetacoplan in October 2020. The companies have global co-development rights for systemic pegcetacoplan. Apellis has exclusive US commercialization rights for systemic pegcetacoplan and retains worldwide commercial rights for ophthalmological pegcetacoplan, including for geographic atrophy. Sobi has exclusive ex-U.S. commercialization rights for systemic pegcetacoplan.
Aurinia Pharmaceuticals formed a collaboration and license agreement in December 2020 with Otsuka Pharmaceutical for the development and commercialization of oral voclosporin for the treatment of lupus nephritis in the European Union, Japan, as well as in the UK, Russia, Switzerland, Norway, Belarus, Iceland, Liechtenstein and Ukraine.
Biogen licensed aducanumab from Neurimmune, a Zurich, Switzerland-based bio/pharmaceutical company. Since 2017, Biogen and Eisai have collaborated on the development and commercialization of aducanumab globally.
Commave Therapeutics has an exclusive worldwide license to develop, manufacture and commercialize KemPharm’s product candidates containing serdexmethylphenidateand d-methylphenidate including Azstarys.
G1 Therapeutics announced in June 2020 a three-year co-promotion agreement with Boehringer Ingelheim for Cosela in small-cell lung cancer in the US and Puerto Rico.
GlaxoSmithKline acquired Tesaro in 2019; AnaptysBio discovered dostarlimab and licensed it to Tesaro.
Merck & Co. has commercial rights to vericiguat in the US, and Bayer has exclusive commercial rights in the rest of world. The companies share equally the costs of the development of vericiguat.
QED Therapeutics and Helsinn formed a collaboration in March 2021 to co-commercialize infigratinib for oncology and all other indications other than skeletal dysplasia indications in the US and equally share profits. Helsinn Group will have an exclusive license to co-develop, manufacture and commercialize infigratinib in such indications outside of the US, excluding China, Hong Kong and Macau.
*Sanofi acquired Kadmon Pharmaceuticals, the developer of Rezurock (belumosudil), in November 2021.
Viiv Healthcare is a specialist HIV bio/pharmaceutical company majority-owned by GlaxoSmithKline with Pfizer and Shionogi as minority shareholders. Viiv Healthcare’s Cabenuva is provided as a co-pack with two injectable medicines: ViiV Healthcare’s cabotegravir and Johnson & Johnson’s rilpivirine.
Source: Center for Drug Evaluation and Research, US Food and Drug Administration







