In the Spotlight: FDA’s Inspections of Foreign Drug Mfg Facilities
The FDA’s inspection process for foreign drug-manufacturing facilities came under scrutiny once again with Congress holding a hearing questioning the frequency and quality of FDA’s foreign drug inspections. What may be next?
FDA’s foreign drug inspections program under scrutiny
The Oversight and Investigations Subcommittee of the Energy and Commerce Committee of the US House of Representatives held a hearing earlier this month (February 6, 2024) to discuss oversight of the US Food and Drug Administration’s (FDA) foreign drug inspection program. The hearing sought to discuss several key issues: (1) the current status of FDA’s foreign drug inspection program; (2) the challenges that FDA inspectors face when conducting foreign drug inspections; (3) the implications of using alternative tools, such as remote inspections, initiated during the COVID-19 pandemic, instead of in-person inspections to oversee drugs made overseas; (4) the frequency and quality of the FDA’s foreign drug inspections compared with those of domestic inspections; and (5) ways in which the FDA can strengthen its foreign drug inspection program.
“I’m concerned that the FDA is failing in its mission. It is not adequately executing its foreign inspection program, which was questionable before the pandemic, became non-existent during the pandemic, and has seen little improvement since,” said House Energy and Commerce Committee Chair Cathy McMorris Rodgers (R-WA) in comments made at the hearing. She also pointed to the differences in the frequency of inspections of foreign drug-manufacturing facilities compared to domestic facilities. “What makes all of this even more disturbing is that in practice we hold domestic manufacturers to much higher standards than we do foreign manufacturers….“We need a level playing field that encourages domestic manufacturing.”
Also testifying at the hearing was Mary Denigan-Macauley, Director of Health at the US Government Accountability Office (GAO), a legislative branch government agency that provides auditing, evaluation, and investigative services for the US Congress. The GAO has issued several reports on the FDA’s inspection process of foreign drug-manufacturing facilities, most recently in 2022, which evaluated the FDA’s program for foreign inspections and concluded that the FDA needs to do more to improve its oversight of foreign drug-manufacturing facilities.
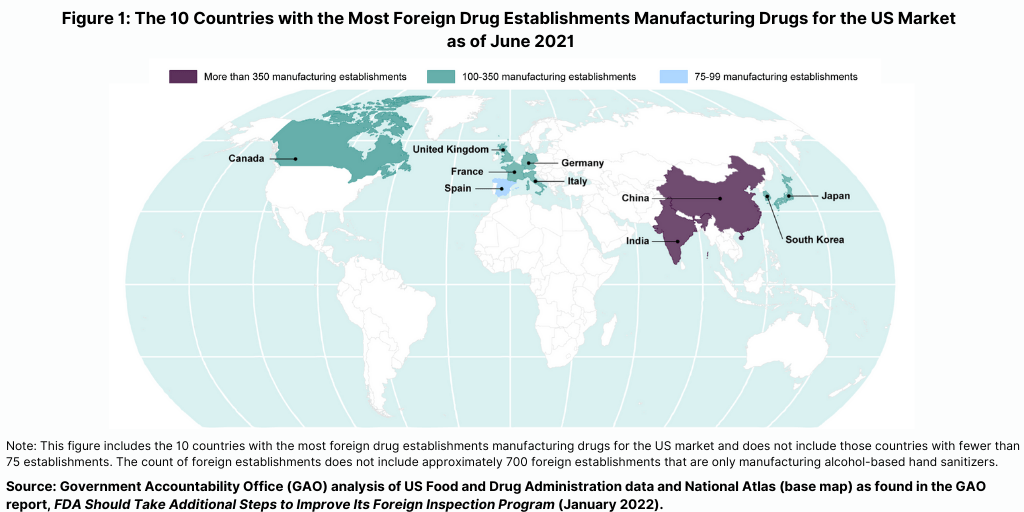
The FDA conducts the largest number of foreign inspections in India and China, where more than one-third of foreign establishments supplying the US market are located (see Figure 1), according to a January 2022 GAO report. The FDA conducts three types of inspections: (1) preapproval inspections, which are conducted prior to when a drug is marketed in the US; (2) surveillance inspections, which are conducted after a drug is marketed in the US to evaluate continued GMP compliance; and (3) for-cause inspections, which are conducted to investigate particular issues, such as those arising from a consumer complaint, specific product-quality and manufacturing issues, and FDA follow-up on prior violations.
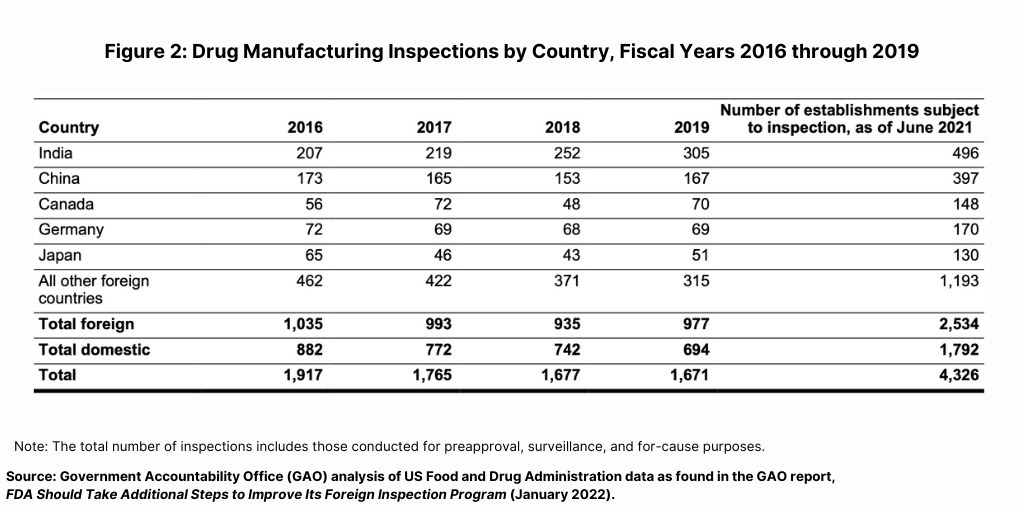
Prior to the COVID-19 pandemic, foreign drug inspections had begun to increase, and the largest increase was in India (see Figure 2). In fiscal year 2019, the FDA began to increase the number of inspections of foreign drug-manufacturing establishments after decreases from fiscal years 2016 through 2018, according to the GAO report. In addition, the FDA continued to conduct more foreign than domestic inspections in each fiscal year from 2016 through 2019. In fiscal year 2019, the FDA continued to conduct the largest number of foreign inspections in India and China, with an increasing number of inspections conducted in India, where about 20% of foreign establishments subject to inspection were located in recent years (see Figure 2).
Progress stalls during and post-pandemic
One of the key concerns raised by certain members of Congress was that FDA’s efforts to increase inspection of foreign drug-manufacturing efforts were stalled because of the COVID-19 pandemic and continue not to progress. Due to travel restrictions and safety concerns raised during the pandemic, the FDA had to pause in-person inspections, both at US domestic drug-manufacturing facilities and foreign drug-manufacturing facilities, and instead used alternative methods, such as remote inspections and record reviews, and in the case of foreign drug-manufacturing facilities, use of inspection information and reporting from foreign regulatory agencies.
“The COVID-19 pandemic made an already bad situation worse,” said House Energy and Commerce Committee Chair McMorris Rodgers. “In March 2020, FDA postponed almost all of its foreign inspections. Although domestic inspections resumed in July 2021, most foreign inspections conducted were still limited only to those deemed ‘mission critical.’ The agency instead resorted to alternative tools, like virtual inspections and record reviews, and relying on inspections conducted by conflicted home country regulators. These tools are no substitute for in-person inspections…”
The impact of the COVID-19 pandemic on FDA’s inspection activity was also addressed by the House Energy and Commerce’s Oversight and Investigations Subcommittee Chair Morgan Griffith (R-VA). “When this Subcommittee last held a hearing on this issue in December 2019, before the known start of the COVID-19 pandemic, there were reasons for cautious optimism that the FDA was taking action to increase the number of foreign inspections and to expand the foreign inspections program to better meet the demands of our global pharmaceutical supply chain,” he said. “FDA’s team of inspectors was almost up to full strength. The number of foreign inspections conducted in India increased from 207 in 2016 to 305 in 2019 with about 70 percent of those inspections being surveillance inspections, he said. “The FDA was implementing mutual recognition agreements with European regulatory agencies that would reduce overlapping inspections and improve inspection record sharing, freeing up resources to focus on high-risk facilities.”
In fiscal year 2019, the FDA conducted the largest number of foreign inspections in India and China, where more than one-third of foreign establishments supplying the US market were located, according to the GAO report. However, beginning in March 2020, FDA postponed most inspections because of the COVID-19 pandemic. “Whatever fitful, halting progress the FDA made towards strengthening its foreign inspection program has been undone by the COVID-19 pandemic and, to date, the FDA has been slow or unable to get foreign inspections back on track,” said the Oversight and Investigations Subcommittee Chair Griffith.
Challenges, progress, and actions to take
A key weakness in FDA’s foreign inspection program cited by certain members of Congress and the GAO is that foreign inspections are pre-announced, something the FDA is in part seeking to improve. Unlike domestic inspections, which are generally unannounced, foreign establishments may receive up to 12 weeks of advance notice of an inspection due to the logistical and access issues to conduct an inspection. “As a result, investigators may be less likely to see the true day-to-day operating environment of foreign establishments as compared to domestic,” said GAO’s Denigan-Macauley.
To address that issue, the FDA implemented a pilot for conducting unannounced inspection at foreign facilities, but some say the results have been mixed. “It is promising to see the FDA implement [its] Foreign Unannounced Inspection Pilot program with both China and India,” said the Oversight and Investigations Subcommittee Chair Griffith. “The goal of this pilot program is to increase the unannounced surveillance inspections of foreign drug facilities. Since March 2022, the FDA has completed 35 unannounced and short-term notice inspections in India. I hope they continue implementing this program and that it will lead to changes within the FDA for foreign inspections. China, on the other hand, is a completely different story. The FDA has not completed any unannounced or short-notice inspection since the pilot program was initiated,” he said.
Staffing issues also are a challenge of the FDA to improve its foreign inspection program. The GAO reported that the FDA had persistent vacancies among investigators available to conduct foreign inspections, which included vacancies among US-based staff who conduct foreign inspections and among staff in FDA’s foreign offices located in China and In India. In line with GAO’s recommendations, GAO’s Denigan-Macauley said that FDA reported in March 2023 that it had developed a plan with six tailored strategies focused on recruitment, development, and retention of drug investigators who perform inspections overseas. “We will continue to monitor FDA’s progress towards implementing it,” she said.
Staffing is also an issue as it relates to the use of translators when conducting foreign inspections. In its January 2022 report, GAO reported that FDA has relied on translators provided by the foreign establishments being inspected, “which investigators told us can raise questions about the accuracy of information FDA investigators collect,” said GAO’s Denigan-Macauley.
Another key issue for FDA is how it will address its backlog of foreign inspections. In its January 2022 report, the GAO noted that the postponement of inspections because of the COVID-19 pandemic led to a backlog of establishments never inspected or not inspected within 5 years—categories for which FDA considers inspections mandatory. “We reported that this backlog could both extend the interval between inspections and reduce the resources FDA has available for other high-priority inspections,” said GAO’s Denigan-Macauley. In line with GAO’s recommendations, the FDA issued a plan for addressing this inspection backlog, which GAO’s Denigan-Macauley said that “we will continue to monitor FDA’s progress towards implementing it.”