Generics and the EU: The Push for Value-Added Medicines
Medicines for Europe, which represents generic-drug and biosimilar manufacturers, is calling on the EU to recognize value-added medicines as a separate group of medicines and with that recognition, to develop specific frameworks for their regulation and reimbursement as a means to encourage innovation of off-patent molecules.
Differences between the US and EU in value-added medicines
Medicines for Europe is calling on officials in the European Union (EU) to establish a regulatory framework that would support the innovation of off-patent molecules akin to the 505(b)(2) regulatory pathway in the US. A 505(b)(2) application is a type of new drug application to obtain the approval of a new drug that contains similar active ingredients to a previously approved drug and is used for changes in dosage form, strength, route of administration, formulation, dosing regimen, or new indication. Medicines for Europe argues that no such similar pathway exists in the EU and is making several recommendations to create a regulatory pathway, set forth intellectual property protection requirements, and establish a reimbursement framework that would support innovation in off-patent molecules. Such measures would establish “value-added medicines” as a separate class of medicine and encourage innovation in formulation and drug delivery, for example, for existing drugs.
“Europe drastically lags behind the United States in terms of supporting value-added medicines,” says Arun Narayan, Head of Global Commercial Development, Viatris, and Chair of the Value-Added Medicines Sector Group, Medicines for Europe, in a February 23, 2021 press statement from Medicines for Europe. “The US has recognized the importance of off-patent innovation for the most challenging chronic diseases like cancer, respiratory conditions and antimicrobial resistance. Rather than stifling this innovation, the US has facilitated it with a dedicated regulatory pathway. Today [February 23, 2021], we are launching our recommendations to put Europe at the center of continuous innovation on off-patent medicines by creating a fit-for-purpose legal framework under the EU Pharma Strategy.”
The European Commission adopted the Pharmaceutical Strategy for Europe in late November 2020, which prioritizes the need for affordable and available medicines that contribute to the sustainability of healthcare. It calls for both legislative and non-legislative action to achieve four main goals: (1) ensuring access to affordable medicines and addressing unmet medical needs (in the areas of antimicrobial resistance and rare diseases, for example); (2) supporting competitiveness, innovation, and sustainability of the EU’s pharmaceutical industry and the development of high quality, safe, effective, and greener medicines; (3) enhancing crisis preparedness and response mechanisms, building diversified and secure supply chains, and addressing medicines shortages; and (4) ensuring a strong EU voice in the world by promoting a high level of quality, efficacy and safety standards.
The strategy’s emphasis on affordable and accessible medicines and innovation supports the move to value-added medicines in the EU says Medicines for Europe. “Re-evaluating the current innovation model should lead to a greater focus on ways to stimulate innovation across the lifecycle of medicines and especially on off-patent molecules,” said Medicines for Europe in a 2021 white paper on value-added medicines.
Recommendations for value-added medicines in the EU
Medicines for Europe is seeking that value-added medicines be recognized as a separate group of medicines in EU legislation and link approval procedures, innovation frameworks, and reimbursement processes “to create an ecosystem that delivers better health to patients, solutions for healthcare systems, and fair returns on R&D investments.” The group says that value-added medicines present an opportunity to innovate off-patent molecules to bring treatments for indications that have no approved therapies and reduce unmet medical need (i.e., through repurposing/repositioning medicines), build on existing medicine to allow for patient-centric design or address healthcare inefficiencies (i.e., through drug reformulation), and combine medicine and different services that can improve treatment outcomes (i.e., through complex combinations). Another advantage of value-added medicines cited by Medicines for Europe is that they build on existing knowledge of a medicine with an already well-established safety profile, which reduces research and development times and lowers cost.
Figure 1 outlines the three main recommendations by Medicines for Europe to support the development of value-added medicines in the EU. These are: (1) design a fit-for-purpose regulatory framework that will enable clarity early in development; (2) recognize value-added medicines as a category of innovation with proportionate incentives; and (3) recognize and define value for healthcare systems. These measures are further outlined below.
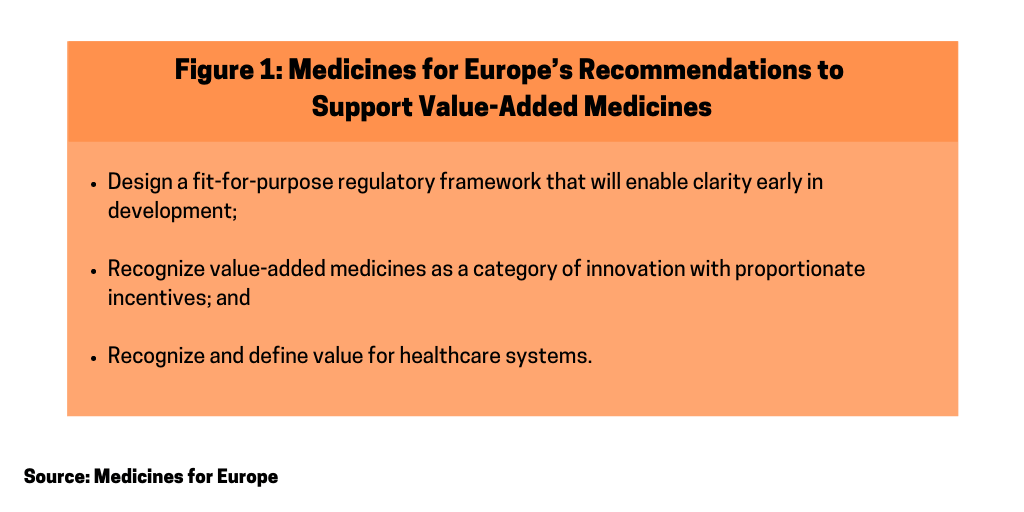
Fit-for-purpose regulatory framework. Medicines for Europe is recommending the establishment of a new legal provision that would result in a dedicated value-added medicine regulatory pathway. Such a dedicated regulatory pathway would include new, pragmatic methods of evidence generation and assessment for affordable and sustainable continuous innovation. This regulatory process would be accompanied by early dialogue with regulators, increased cooperation between all healthcare stakeholders, and fit-for-purpose scientific advice, so that developers would be able to gain clarity and invest in value-added medicines.
Recognition of value-added medicines as a separate category of medicines. Medicines for Europe is recommending that value-added medicines be recognized as a separate category of innovation with incentives to encourage further development of value-added medicines in Europe. The group is proposing that, for the innovation on well-established substances, a noncumulative period of four years of data exclusivity be granted, subject to certain conditions and provided that the additional value that is delivered by the innovation can be demonstrated by appropriate data.
Medicines for Europe says that the purpose of this proposal is to stimulate innovation where it is not currently encouraged but not to use the measure to further extend exclusivity for medicines that have already benefitted from exclusivity period. “We, therefore, consider that the global marketing authorization (GMA) concept should continue to apply and that data protection would not apply to data that was previously used within the GMA (i.e., no further protection for studies already benefiting from exclusivity). The proposed framework should not facilitate the delay of the off-patent competition.” The group is calling for safeguards to achieve this balance.
Adoption of an evaluation framework for value-added medicines. Medicines for Europe is also calling for the adoption of an evaluation framework for value-added medicines in the reimbursement process. It says that the decision-making process should consider relevant value dimensions that demonstrate the benefits of value-added medicines in different purchasing/procurement mechanisms that should be defined with healthcare stakeholders: regulators, patients, healthcare professionals, and payers. It is recommending that pricing and reimbursement rules be shaped to adequately assess continuous innovation and be adjusted to the specificity of value-added medicines with different rules and a different assessment process than the current pathways for generic medicines (e.g., internal price referencing, mandatory discounts) or innovative medicines (e.g., clinical benefit) as it says that these are not appropriate for value-added medicines.
Rationale for change
Medicines for Europe is recommending these measures as it says that the current approaches to stimulate innovation of off-patent medicines have not been successful, the current approval process is too cumbersome, and the reimbursement process is not adequate. Further details are outlined below.
Past approaches to stimulate innovation. Medicines for Europe says that such measures are needed to effectuate innovation in off-patent molecules as past approaches have not been successful. “Multiple attempts to foster innovation on well-established (often off-patent) molecules have failed in Europe[;][e]ven in clearly designated priority areas of the current legislation, such as pediatric indications,” said Medicines for Europe in its 2021 white paper. The group points out that pediatric-use marketing authorization measures introduced incentives to stimulate development of off-patent molecules but did not deliver sufficient results as it had been used only six times in this way. “By recognizing VAMs [value-added medicines] in their own right, the EU can remove the barriers to continuous innovation,” said Medicines for Europe in its 2021 white paper.
Current approval process. The group further says the current approval process is too cumbersome. At present, there is no specific regulatory pathway for value-added medicines, something that Medicines for Europe is seeking. “Several legal bases can apply for Value Added Medicines to be registered in the EU,” said Medicines for Europe in its 2021 white paper. “The consequence is high complexity for the applicant–different pathways require different levels of evidence and developers often need to seek regulatory advice to determine which legal pathway would be most suitable for their product. This wastes resources and adds to the complexity and cost of the development. As continuous innovation is based on well-established substances, regulatory requirements should be further tailored to provide clarity for VAM [value-added medicine] developers by increasing predictability of required evidence and development costs and regulatory timelines.”
Medicines for Europe further says that the current system does not encourage innovation for off-patent molecules and new measures are needed. “The current legislative framework encourages innovation in new chemical or biological entities and research for rare diseases or subsets of diseases. The EU pharmaceutical legislation also encourages follow-on competition at patent expiry with clear regulatory and market pathways for generic and biosimilar medicines, thereby massively increasing patient access to medicines,” said Medicines for Europe in its white paper. “However, the development of Value-Added Medicines requires additional evidence generation when compared to traditional follow-on products, with further complexity, cost and prolongation of the development. Existing regulatory incentives in the EU framework, such as one year of non-cumulative data exclusivity for a new indication on a well-established substance have failed to stimulate more development—this pathway has never been used. Value-added medicines innovation is not sufficiently encouraged under either the generic, biosimilar or originator framework.”
Current reimbursement of value-added medicines. Medicines for Europe also says there is confusion in EU markets regarding the fair valuation of value-added medicine innovation. “The value assessment process is heterogeneous across EU Member States, and Value Added Medicines are often categorized as generic medicines because the innovation is on an off-patent molecule, with no process/framework in place to recognize their additional value in a proportionate way,” said Medicines for Europe in its 2021 white paper. “In other instances, VAM [value-added medicine] manufacturers are faced with requests for evidence in HTA [health technology assessment] processes that are designed for originator pharmaceuticals and, therefore, demand disproportionate evidence generation which is not fit for purpose and prevents affordable innovation from reaching patients.” By establishing an EU-level recognition of value-added medicines, Medicines for Europe says that EU member states can develop proportionate mechanisms to reward this kind of innovation.






