FDA Advances Quality Management Ratings System for Pharma
The FDA is developing a framework to objectively rate the Quality Management Maturity of pharmaceutical manufacturing sites. How would such a rating system be used by regulators, bio/pharma companies, suppliers, and other stakeholders?
The FDA’s Quality Management Maturity (QMM) Program
To increase transparency and incentivize investment in pharmaceutical manufacturing, the Office of Pharmaceutical Quality in the Center for Drug Evaluation and Research (CDER) at the US Food and Drug Administration (FDA) is developing a framework to objectively rate the Quality Management Maturity (QMM) of pharmaceutical manufacturing sites. A QMM ratings system is part of CDER’s larger vision to achieve a new paradigm for pharmaceutical manufacturing in the 21st century to be maximally efficient, agile, and flexible for producing high-quality pharmaceutical products. It goes beyond simply meeting minimum current good manufacturing practice (CGMP) standards and moves closer to robust quality management systems. The FDA issued a white paper last month (March 2022) on the QMM program and will be holding two workshops in May (May 2022) to gain further input from stakeholders.
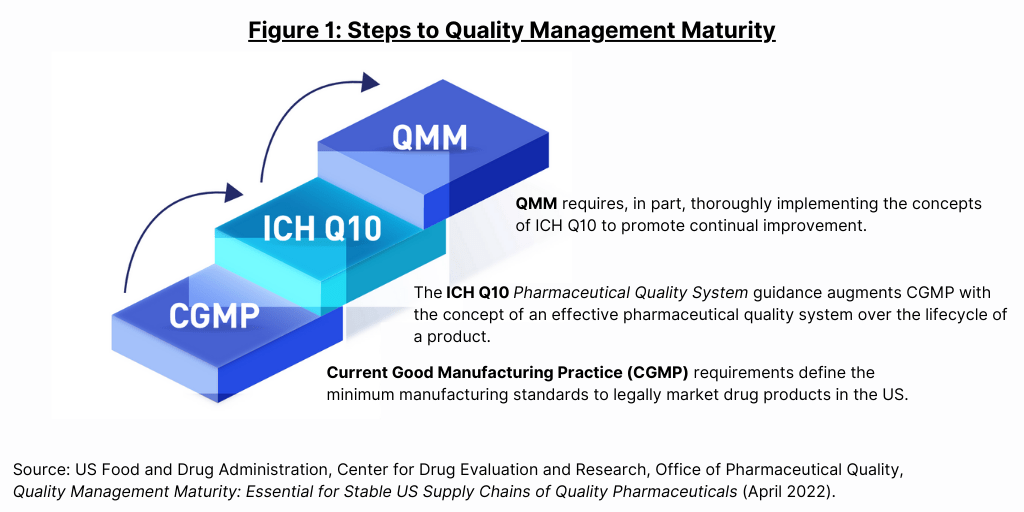
The idea for a QMM rating system was part of a proposal by the FDA’s Drug Shortages Task Force to address the problem of drug shortages by using a rating system to incentivize drug manufacturers to invest in achieving Quality Management Maturity (QMM). As FDA specifies in its white paper: “QMM is the state attained by having consistent, reliable, and robust business processes to achieve quality objectives and promote continual improvement.” Gauging QMM requires, in part, determining how well and how thoroughly a drug manufacturer has implemented the concepts of ICH Q10, Pharmaceutical Quality System, an internationally harmonized guidance to assist pharmaceutical manufacturers in developing effective quality management systems (see Figure 1).
In its white paper, the FDA says that a transparent rating system could inform purchasers about the level of QMM at sites from which they purchase drugs. “In the absence of the transparency generated by ratings of QMM, there is risk that price competition and cost minimization will continue to be key market drivers, especially for generic drugs, without direct reward for manufacturers who actively invest to avoid future shortage,” the white paper said. “Some pharmaceutical firms have been slow to implement robust, mature quality systems and adopt the quantitative measures of quality that have been successful in the automotive and aerospace industries. Transparent QMM ratings could empower manufacturers to identify ways to improve the effectiveness of their PQSs [pharmaceutical quality systems], realize regulatory flexibilities described in ICH Q10, and help move the pharmaceutical industry toward the Six-Sigma quality common in other industries.” The agency also says that mature quality management assures that quality product is on the market at entry and over the product’s entire lifecycle, asserts the FDA in its white paper.
Challenges to the FDA in developing a QMM program
The primary stakeholders potentially impacted by a QMM rating program comprise the “6 Ps” of the pharmaceutical supply chain: pharmaceutical manufacturers, purchasers, payors, pharmacies, providers, and patients. Stakeholder engagement has been a critical element in developing a CDER QMM program. In February 2020, CDER sponsored and participated in a workshop held by the Duke-Margolis Center for Health Policy at Duke University on Understanding How the Public Perceives and Values Pharmaceutical Quality. Stakeholders represented at this workshop includedpatients, healthcare providers, purchasers, pharmacies, and payors. These stakeholders identified three key areas for future work and collaboration across the stakeholdercommunity: (1) assessing perceptions surrounding quality to improve communication,(2) improving transparency, and (3) developing quality ratings.
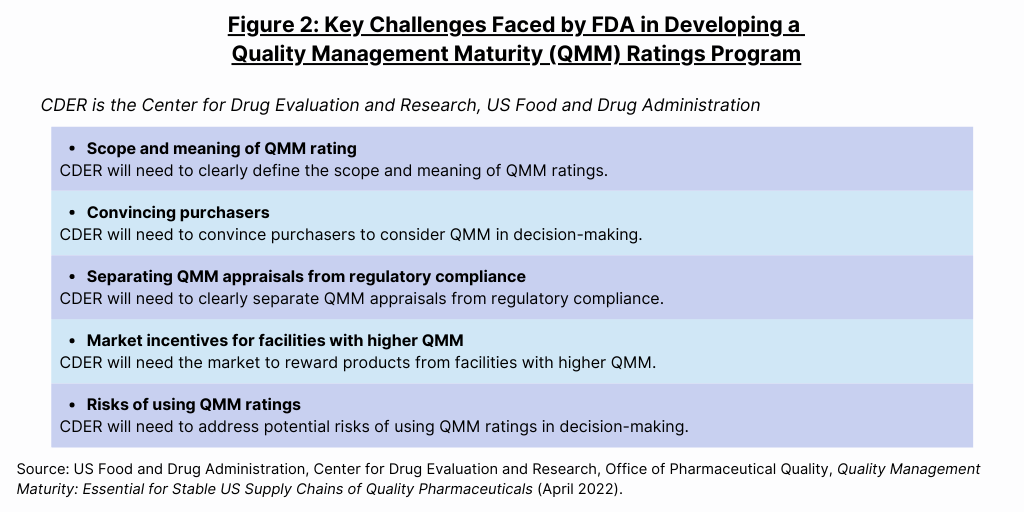
“Operationalizing a QMM rating program for pharmaceutical manufacturers requires a collaborative and transparent partnership between FDA, industry, and other stakeholders,” said the FDA in its white paper. The agency formed a multidisciplinary, multi-Center Working Group to facilitate the development of a QMM ratings program. This working group is developing a framework to objectively assess and rate the QMM of manufacturing sites using interactive site engagement along with surveillance intelligence. In developing the framework, the FDA is considering standardized assessment tools, policy approaches, industry incentives, transparency, and communications. As CDER has started to develop the QMM program, engaged stakeholders have started to identify key challenges to overcome in order to realize a successful program (see Figure 2).
Key elements of a QMM rating program
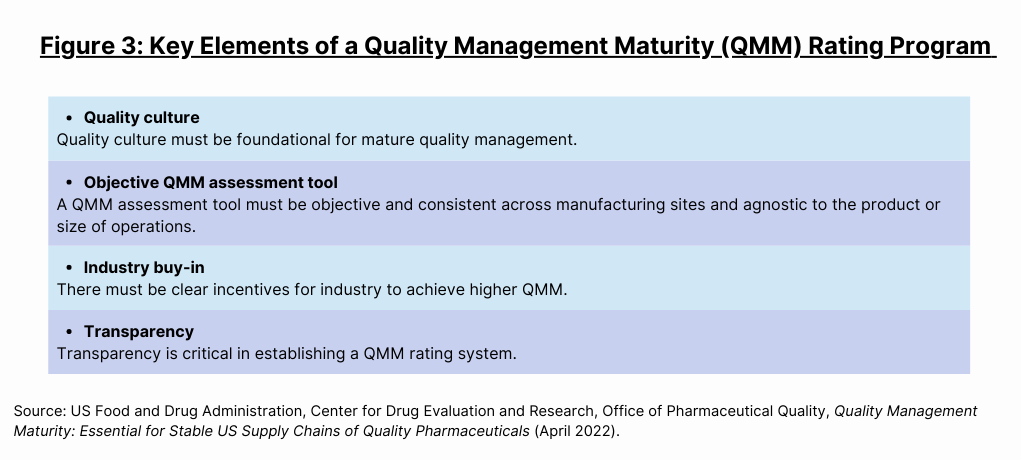
In its white paper, the FDA notes that “[c]hanging the course of pharmaceutical quality by using a QMM rating system is a move toward performance-based regulation of the pharmaceutical industry.” It says that most, if not all, key challenges can be overcome. By engaging stakeholders, the agency has identified what will be needed in a QMM rating program (see Figure 3).
Benefits to stakeholders
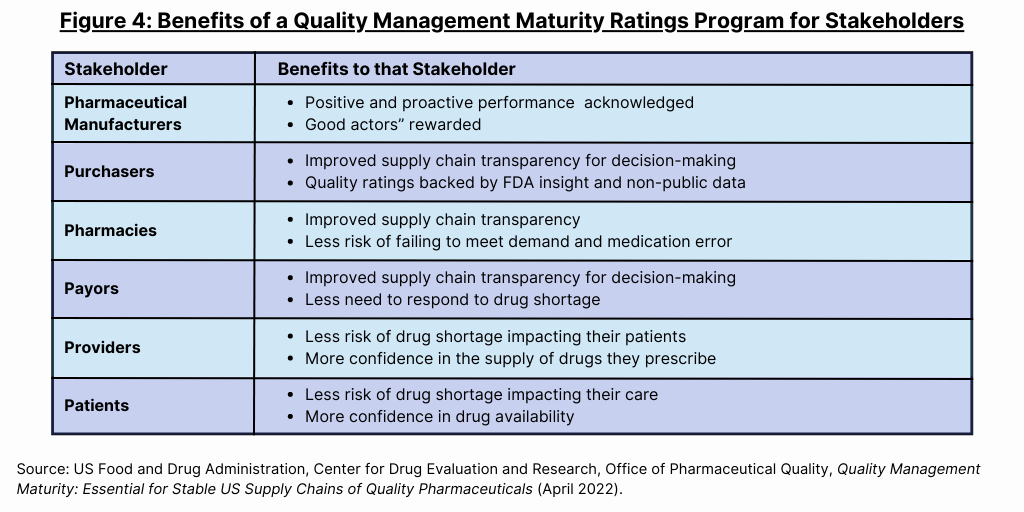
In its white paper, the FDA say that a QMM rating program that overcomes key challenges and includes key elements will provide benefits for all stakeholders (see Figure 4) as well as benefits to the FDA. “Minimally, purchasers and payors will get more insight into the supply chain of the drugs they buy or reimburse, pharmaceutical companies will get more insight into the robustness of their supply chains, and patients, pharmacies, and healthcare professionals get improved clinical care via medicine less at risk of quality-driven shortage, said the FDA.
The FDA has conducted two pilot programs with pharmaceutical manufacturers to inform the criteria used to objectively measure a manufacturing site’s QMM. One pilot is focused on domestic manufacturers of finished dosage form products, and the other on foreign manufacturers of APIs. Feedback from the participants in the pilot programs is now helping determine best practices for conducting the assessments, the assessment tool, and associated logistics. “Minimizing the burden on manufacturing sites during QMM evaluations is an important consideration in the development of a QMM program,” said the FDA in its white paper.







