EU Launches New Critical Medicines Alliance
The European Commission has launched the Critical Medicines Alliance, an EU-level initiative to develop recommendations and provide advice to EU policy decision-makers on how to address long-standing medicines shortages, including ways to shore up domestic manufacturing in the EU and strengthen the bio/pharma supply chain.
Launch of the Critical Medicines Alliance
The European Union (EU) took a major step this week (January 16, 2024) to further address drug shortages in the EU and the related supply lines with the launch of the Critical Medicines Alliance. First proposed by the European Commission last October (October 2023), the Critical Medicines Alliance is bringing together all relevant stakeholders to strengthen cooperation between the European Commission, national governments, industry, and civil society to address drug shortages in the EU and security of supply. It will identify the challenges, priorities for action, and possible policy solutions to the issue of shortages of critical medicines in the EU. The Alliance is a consultative mechanism that will also act as a network to accelerate the delivery of EU action in this field and will be charged with developing recommendations and providing advice to the European Commission, EU member states and other EU decision-makers on how to address long-standing medicines shortages.
To gain the participation of those stakeholders, the European Commission’s Health Emergency Preparedness and Response Authority (HERA), this week (January 16, 2024), launched an open call for those interested parties to join the Critical Medicines Alliance with a deadline for applications one month later on February 16, 2024. The applications will then be reviewed in February and March (February and March 2024) with the Alliance holding its first meeting and beginning its work in late April 2024. The Alliance will remains open to new members at all stages of its operation. However, the first deadline for application is important as it will be used by the European Commission to determine who will be able to participate in the first steps of the Alliance.
The basis of the Critical Medicines Alliance was first put forth by the European Commission last October (October 2023) in a Communication presenting actions to better prevent and mitigate critical medicine shortages in the EU for this winter and beyond. In launching the Alliance, the European Commission said that recent critical shortages, including of certain antibiotics, show that continued coordinated action is needed to make Europe’s medicine supply chains more resilient in the long run. The Communication placed a particular focus on the most critical medicines to which it prioritized security of supply in the EU. The key objective of the Alliance is to provide an inclusive and transparent consultative platform to the European Commission and other EU decision-makers. It focuses on identifying industrial challenges and determining the most suitable actions and tools to effectively address vulnerabilities in critical medicines supply chains.
The Alliance builds on recent action by EU authorities to address drug shortages in the EU. Last month (December 2023), the European Commission, the Heads of Medicines Agencies, and the European Medicines Agency published the first version of the list of critical medicines for the EU/European Economic Area (i.e. “the Union list”), which contains more than 200 active substances of medicines considered critical for healthcare systems across the EU/European Economic Area, for which continuity of supply is a priority and for which shortages should be avoided. The list contains active substances of innovator and generic drugs covering a wide range of therapeutic areas and includes vaccines and medicines for rare diseases. It reflects the outcome of a review of 600 active substances taken from six national lists of critical medicines. The list will be expanded in 2024 and will then be updated every year.
The work of the Alliance focuses on critical medicines that face the greatest vulnerabilities. The European Commission is currently conducting a vulnerability analysis for a first sub-set of substances listed on the Union list of critical medicines. The Alliance will work on that subset of substances before tackling the rest of the Union list, by pooling the expertise and resources of its members to determine how vulnerabilities in the supply chains could be best addressed. The European Commission’s pilot exercise will first analyze the vulnerabilities in the supply chains of an initial set of 10-20 substances from the Union list of critical medicines. The Alliance’s efforts will initially focus on the outcomes of this analysis, expected to be published in April 2024. Once the pilot exercise is finalized, the European Commission will proceed with evaluating the remaining medicines in the Union list. It will then recommend priority actions for the near future and propose new tools to address the challenges it has identified. In particular, the recommendations will focus on mitigating structural risks, reinforcing supply by making demand more predictable, encouraging diversification, and boosting manufacturing.
Manufacturing and the supply chain: a key focus
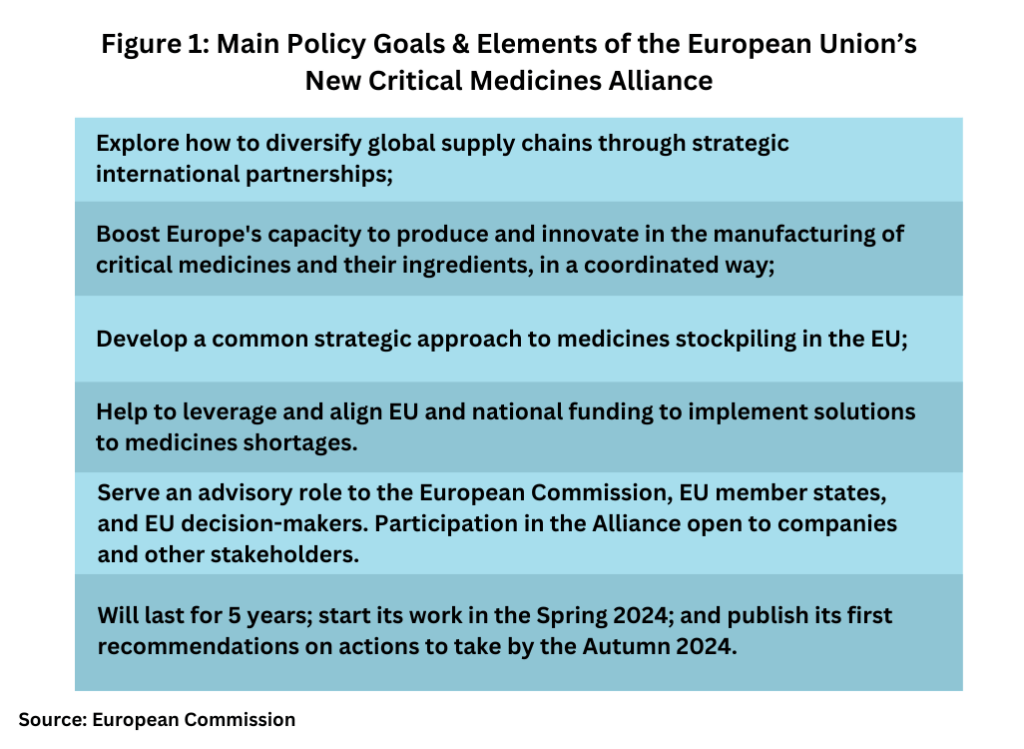
Shoring up domestic manufacturing in the EU and better securing the supply chain for those critical medicines is a key focus of the Alliance. It will build on the experience from other EU alliances that address major industrial challenges (such as the Batteries and Critical Raw Materials Alliances) and use a variety of policy measures as outlined below (see Figure 1):
- Exploring how to diversify global supply chains through strategic international partnerships;
- Boosting Europe’s capacity to produce and innovate in the manufacturing of critical medicines and their ingredients, in a coordinated way;
- Developing a common strategic approach to medicines stockpiling in the EU;
- Helping to leverage and align EU and national funding to implement solutions to medicines shortages.
Alliance part of an overall EU healthcare strategy
The launch of the Critical Medicines Alliance builds on the work under the European Health Union, an EU policy initiative, which has an overall aim of better protecting the health of EU citizens, equipping the EU and its member states to better prevent and address future pandemics, and improve the resilience of Europe’s health systems. These policy actions notably include the reinforced mandate of the European Medicines Agency and the recently published pharmaceutical reform. It follows a call by EU member states at a June 2023 meeting of the European Council, later confirmed in October 2023, and from the European Parliament.
“Today’s [January 16, 2024] launch of the work on a Critical Medicines Alliance is another chapter for our European Health Union,” said Stella Kyriakides, the European Commissioner for Health and Food Safety, in commenting on the launch of the Alliance. “It marks the beginning of a concerted push to bolster and modernize the production of critical medicines in the EU and diversify international supply chains. As a new industrial pillar of our strong European Health Union, the Alliance will help to change the way we produce and procure medicines and ultimately reinforce our security of supply. With this initiative, we will ensure that governments, industry, health professionals and civil society work closer together than ever before to identify solutions to ensure patients are better protected and always have access to the medicines they need.”

Participation and implementation plan
The Critical Medicines Alliance is open to all companies and organzations, EU member states, local and regional authorities and their agencies, social partners, civil society, health professionals, patients, consumers as well as other stakeholder groups, EU bodies, and agencies.
In terms of governance, the Steering Board of the Alliance will provide strategic orientation to the work of the Alliance. It will be comprised of representatives from EU member states (Ministries of Health and Industry), the industry, patients and healthcare professionals, the European Medicines Agency’s Medicine Shortages Steering Group, and the European Medicines Agency itself as well as the chairs of thematic Working Groups and representatives of the European Commission, acting as secretariat.
The Alliance will last for five years. It is expected to start its work this coming spring (Spring 2024) and publish its first recommendations on actions to improve the supply of critical medicines by this autumn (autumn 2023).
After the adoption of the rules of procedure during the first meeting of the Alliance Forum in the second quarter of 2024, Alliance members will start their collaboration in thematic Working Groups. While the Working Groups are still to be determined, they are likely to include the strengthening of EU manufacturing capacities, strategic stockpiling, procurement, diversification of supply, and international partnerships and cooperation.
The recommendations of the Working Groups will form the basis of a Strategic Plan, a multi-annual set of actions with proposed milestones and corresponding deadlines drafted by the Steering Board and endorsed by the Alliance Forum. The Strategic Plan will guide the work of the European Commission, EU member states and other EU decision-makers if they decide to implement the proposed actions. The Alliance Forum will evaluate the Strategic Plan every two years to ensure it remains adapted to effectively support the strengthening of critical medicines supply chains and active pharmaceutical ingredients supply chains. Additionally, the European Commission may consult the Alliance Forum on other pertinent policies related to the outlined focus or the broader critical medicines sector.







