Thermo Fisher Investing $475 M to Expand Capacity and Capabilities
 |
|
Michael Shafer |
Thermo Fisher Scientific is investing more than $475 million in 2020 in commercial capabilities and capacity for biologics, cell and gene therapies, and drug products. Michael Shafer, Senior Vice President and President, Pharma Services, Thermo Fisher Scientific, detailed the expansions at the DCAT Member Company Announcement Forum—Virtual Edition, a special online forum of the major news announcements from DCAT member companies originally to be made March 23, 2020 at DCAT Week ‘20, in New York.
Shafer explained that the $475-million investment in 2020 is in addition to those made in 2019 and brings the total investment by the company over two years to approximately $800 million across the company’s pharma services business. Figure 1 highlights the key investments being made in development and manufacturing services for biologics, cell and gene therapies, and drug products.
In biologics, Thermo Fisher plans to open a new Bioprocessing Collaboration Center in St. Louis later this year, where multiple Thermo Fisher businesses will jointly develop bioprocessing products, workflows, and services. The company completed a $50-million expansion at its St. Louis site last year (2019) to double production capacity for biological drug-substance development and commercial manufacturing.
In addition, the company will open a new cell-therapy development and manufacturing collaboration center in Princeton, New Jersey to combine pharma services and biosciences expertise from across the broader Thermo Fisher network. A new cell-therapy facility will come on line at the same location later this year (2020). Following its $1.7-billion acquisition of Brammer Bio, a cell- and gene-therapy CDMO in 2019, Thermo Fisher has expanded its viral vector development and manufacturing capabilities by opening a new site in Lexington, Massachusetts and expanding sites in Cambridge, Massachusetts and Alachua, Florida.
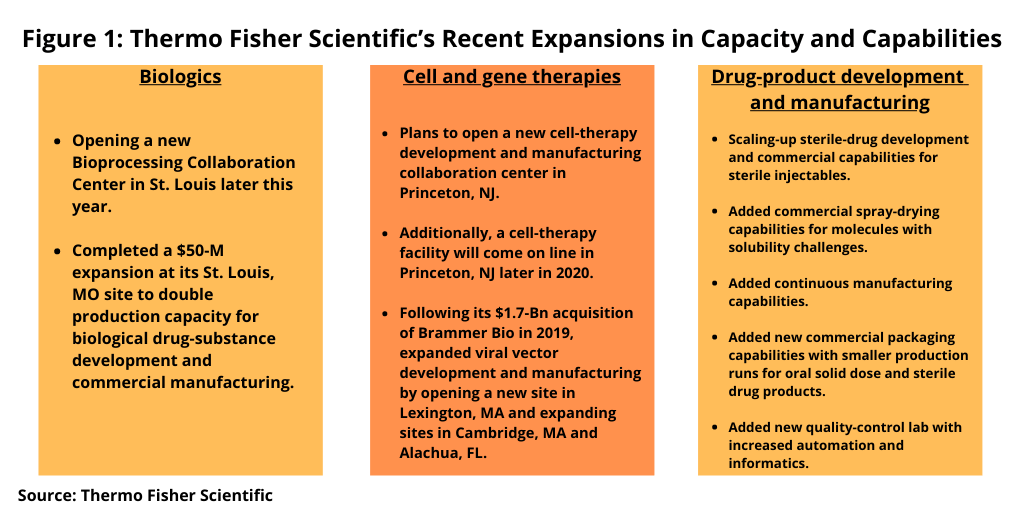
On the drug-product side, Thermo Fisher is making investments to scale up its sterile drug development and commercial capabilities to cover a range of dosage forms for sterile injectables. In addition, the company is now providing commercial spray-drying capabilities for molecules with solubility challenges. The company is also providing oral solid dose continuous manufacturing and is planning additional investment in a second continuous manufacturing line that will be designed for high-volume commercial oral solid dose products and be capable of producing 1.5 billion to 3 billion tablets per year.
In addition, the company is adding new commercial packaging capabilities with smaller production runs for oral solid dose and sterile drug products. In addition, the company invested in a new quality-control lab with enhanced analytical, automation, and informatics capabilities to integrate and automate workflows by combining capabilities across the company. The laboratory features 20,000 square feet of completely new space. More than 50 chemists, data reviewers and managers will be working in the model lab.

