Applications of Liquid-Filled Capsules for Challenging APIs in Pharmaceutical Manufacturing
Liquid-filled capsules offer significant benefits for active pharmaceutical ingredients (APIs) presenting formulation or manufacturing challenges. Their use is beneficial throughout the different phases of drug development, from preclinical to commercialization. How can you benefit?
Introduction
Liquid-filled capsules (LFCs) are an efficient drug-manufacturing option that offer significant benefits for active pharmaceutical ingredients (APIs) presenting formulation or manufacturing challenges. These include ease of scalability and manufacturing, faster absorption, and higher product stability. They also offer abuse-deterrent benefits because they make it harder to insufflate, inject, or alter the extended-release properties of potent drugs. Their use is beneficial throughout the different phases of drug development, from preclinical to commercialization.
In this article, we discuss the process of liquid-filled capsule manufacturing, and allow you to determine if LFCs are the better choice for your API over other solid dosage forms.
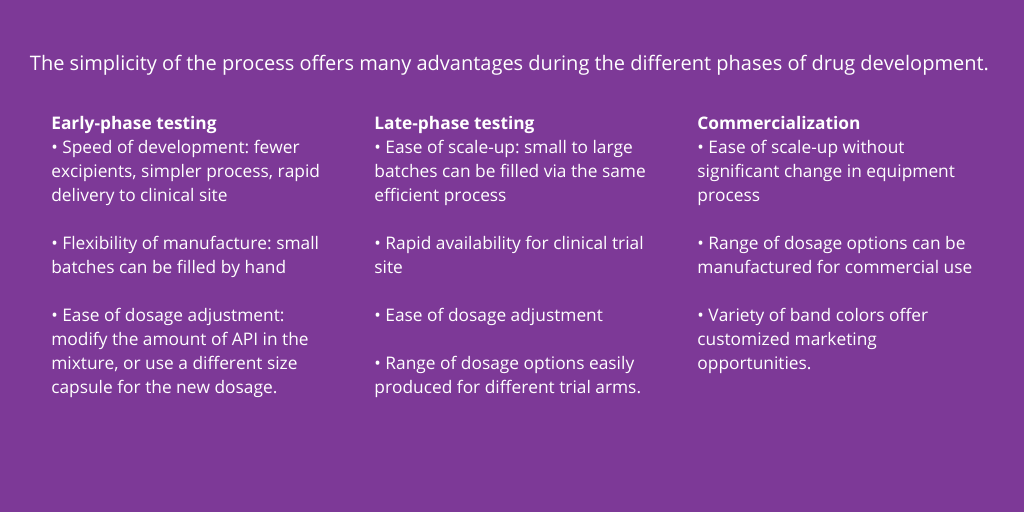
Manufacturing Process
The encapsulation of drugs as liquid-filled capsules is a relatively simple and efficient process. Gelatin, largely sourced from collagen, is the base material for the shell matrix of liquid-filled capsules. Vegetarian options include a polymer, such as hydroxypropyl methylcellulose (HPMC), potato starch, and carrageenan from seaweed.
The API is suspended or dissolved inside a heated, jacketed kettle via mixing with excipients as needed. The excipients are chosen based on the properties of the API, and typically include triglycerides, mixed glycerides, pharmaceutically acceptable co-solvents, such as polyethylene glycol, and propylene glycol, water-soluble and water-insoluble surfactants, solubility enhancers, and other additives such as α-tocopherol. These excipients can help improve bioavailability, ensure equal distribution of the active ingredient throughout the mixture, and keep the drug substance contained.
The formulation is delivered to filling machinery through heated hoses, if necessary, to maintain optimal temperature, and is used to fill two-piece, hard-shell capsules. The capsules come in a variety of sizes, depending on the mixture and API concentration being developed. Once filled, the capsules are sealed to prevent leaking, usually with a colored band. When cooled to room temperature, the mixture solidifies to a wax-like consistency, except in the case of vitamin E oil or other fully liquid products.
Band sealing offers several benefits:
- Does not require elemental impurities testing as there is no addition of alcohol
- Faster commercial equipment is available for band sealing
- Band seals provide tamper evidence for over-the-counter drugs
- Unique marketing/brand differentiation options are available through choice of band color.
Ideal Drug Candidates

Poorly water-soluble
The oral absorption of a drug is directly related to its water solubility and gastrointestinal permeability. In recent decades, updated screening methods and bioavailability enhancements have led to increasing numbers of poorly water-soluble small molecules being considered for development — liquid fill technology is ideal for new molecular entities (NMEs) classified as having low solubility and high permeability.
For instance, candesartan is a highly potent drug that is poorly absorbed when administered as tablets. Deepthy and Murthy (1) used liquid-filled, hard-shell capsules to improve the bioavailability of the prodrug candesartan cilexetil, using different surfactants and excipients. The study determined that the encapsulation of candesartan cilexetil with sodium lauryl sulfate as a surfactant, and other excipients in the formulation, significantly influences in vitro drug release.
A study published in the journal, Pharmazie, confirmed the efficiency of liquid-filled, hard-shell capsules and the appropriate carrier material “… to improve peroral bioavailability of the very poorly water-soluble hepatoprotectant silymarin through formation of a semisolid dispersion (SD) system…” (2)
A related but somewhat different option to improve solubility of an API is to reduce the particle size via nano milling, develop a suspension for the nano-milled particles, and deliver in liquid-filled capsules. (3)
Low dose/high potency
Compounds in the low dose/high potency category include Schedule I drugs, hormones, and cytotoxic APIs. These products present two main challenges for the manufacture of solid dosage forms such as tablets:
- Content uniformity. When working with low-dose APIs in solid format, ensuring that each tablet has the same quantity of active ingredient is challenging. By dissolving the active ingredient and creating a solution, improvements in unit-dose homogeneity are realized. In a Japanese study, 100% of the capsules investigated met the requirement for content uniformity. (4)
- Containment of potent drug material during processing. Highly potent APIs (HPAPIs) are increasingly numerous in development, with more classes of molecules and new chemical entities being formulated for a broad range of conditions. Liquid-filled capsules provide a safer alternative to powders, in that the HPAPI powder is dissolved in the confines of a closed system, and operators are not exposed to airborne particles during the manufacturing process. (5)
To demonstrate the containment of API during capsule filling, a semi-solid formulation of phenacetin (3-mg dose) was used as a model compound. (6) Swab tests were conducted on the surfaces of different parts of the filling machinery after a filling operation. The results demonstrated that no API was found on any of the machine bushings of the capsule-filling equipment, a result that would not be possible when filling with powder. The lower detectable limit of the analytical method was 0.25 μg. This result demonstrated that the incorporation of potent APIs into liquid-fill materials can drastically reduce the exposure of CDMO machine operators to fine particles of HPAPIs.
Low melting point
During the drug manufacturing process, heat is created, and a solid-form API with a low melting point is likely to stick to the equipment, which creates processing and stability issues, as well as unnecessary waste or loss of API. In this scenario, a liquid formulation that takes advantage of the low melting point, filled into capsules, effectively eliminates such issues.
Commercially available products which fall into this category include fish oils, vitamins A and E, and phospholipids.
Critical stability profile
Drug stability can be negatively impacted by environmental conditions during processing, particularly when there is high humidity that may be absorbed by the drug product. The antibiotic vancomycin is a good example, with many different formulation options (7). Vancomycin hydrochloride is hygroscopic and freely water-soluble, and will absorb large amounts of moisture if not packaged appropriately. When vancomycin first arrived on the market, each unit dose was dispensed in a glass vial, and reconstituted immediately prior to use. In the 1980s, vancomycin was incorporated into a polyethylene glycol (PEG) matrix filled into hard gelatin capsules, which protected the drug product from moisture and produced a convenient, stable dosage form. The hard-shell capsule formulation demonstrated systemic equivalency to the solution of the API. (8)
Additional benefits
Unlike softgels, hard-shell gelatin capsules offer the possibility of combination filling. Drugs can be encapsulated in the form of beads, micro tablets, and pellets, in combination with the liquid formulation. In this way, you can incorporate multiple previously incompatible drugs in a single capsule, in a formulation that is effective, stable, and esthetically pleasing to consumers.
Because of these qualities, LFCs offer a great opportunity for line extensions, and brand differentiation, in the competitive pharma market. LFCs are an ideal option for reintroducing existing products in a new format to revitalize the brand. Reformulating for faster onset of action, or better efficacy, or to combine two existing APIs into a new drug product, are all opportunities to get more mileage out of existing molecules.
Final Considerations
LFCs have the potential to transform formulation challenges into simple solutions. They offer a highly marketable dosage form due to their capability to encapsulate different combinations of tablets, pellets, caplets, and capsules in liquid. Last but not least, they offer patient benefits with increased bioavailability and sustained release drug delivery.
Choosing Your Partner
As an integrated CRO/CDMO, Altasciences can quickly determine if your API has the attributes to benefit from a LFC dosage form, and get your drug to market faster.
By partnering with Altasciences, you can leverage our unique capabilities in the formulation, development, and manufacturing of drug products for small molecules, in addition to our industry-leading expertise in liquid-filled capsules.
We offer:
- CRO/CDMO solutions to take your molecule from formulation, to early phase clinical trials, all the way through to commercialization
- A state-of-the-art facility with:
- Formulation and analytical laboratory
- cGMP manufacturing suites
- cGMP high-potency area with segregated Class C manufacturing area
- cGMP warehouse
- Narcotic storage area (DEA License for Schedules I-V)
- Extensive in-house experience developing and manufacturing LFCs with a 97% right first-time batch release success rate
- On-site ICH stability storage and testing
- Controlled substance testing
- Drug-product release testing
- Multiple dosage forms
- Advanced equipment and banding/sealing technology.
To discover our full range of manufacturing solutions, visit altasciences.com or contact one of our experts today.
About Altasciences
Altasciences is an integrated drug development solution company offering pharmaceutical and biotechnology companies a proven, flexible approach to preclinical and clinical pharmacology studies, including formulation, manufacturing, and analytical services. For over 25 years, Altasciences has been partnering with sponsors to help support educated, faster, and more complete early drug development decisions. Altasciences’ integrated, full-service solutions include preclinical safety testing, clinical pharmacology and proof of concept, bioanalysis, program management, medical writing, biostatistics, clinical monitoring, and data management, all customizable to specific sponsor requirements. Altasciences helps sponsors get better drugs to the people who need them, faster.
References
- Y Deepthi, T E Gopalakrishna Murthy, “Design and Development and Evaluation of Candesartan Cilexetil Liquid Filling Formulations”, Int J Pharm Investig. Apr-Jun 2015;5(2):81-6, accessed Sept. 8, 2021.
- A Hussein, S El-Menshawe, M Afouna, “Enhancement of the in-vitro Dissolution and in-vivo Oral Bioavailability of Silymarin from Liquid-Filled Hard Gelatin Capsules of Semisolid Dispersion using Gelucire 44/14 as a Carrier” Pharmazie. 2012 Mar;67(3):209-14, accessed Sept. 8, 2021.
- Meng Li, Mohammad Azad, Rajesh Davé, and Ecevit Bilgili, “Nanomilling of Drugs for Bioavailability Enhancement: A Holistic Formulation-Process Perspective,” accessed Sept. 8, 2021.
- N Katori, N Aoyagi, S Kojima, “The Study of the Applicability of Content Uniformity and Weight Variation Test—The State of Commercial Tablets and Capsules in Japan,” accessed Sept. 8, 2021.
- Cynthia A. Challener, “HPAPI Complexity Prompts New Delivery Mechanisms,” accessed Sept. 8, 2021.
- W.J. Bowtle, S.L. Burrows, E.D. Holmes, B.E. Jones, S.S.M. “Formulations and Capsules: an Improved Way to Dandle Toxic Compounds,” AAPS Annual Meeting and Exposition, Pharm. Res. 7 (1989) 612 (Poster PT).
- Vancomycin Product Circular, accessed Sept. 8, 2021.
- R.A. Lucas, W.J. Bowtle, R. Ryden, “Disposition of Vancomycin in Healthy Volunteers from Oral Solut7.ion and Semisolid Matrix Capsules,” J. Clin. Pharm. Ther. 12 (1987) 27–31, accessed Sep. 8, 2021.



