Novel Excipients: Gaining Traction in the Industry
The FDA is considering a pilot program for the toxicological and quality evaluation of novel excipients. Excipients now are not separately reviewed by the FDA, and some say a separate review mechanism would support development of new excipients and overall product innovation.
Novel excipients and the FDA
Currently, novel excipients are not precluded a regulatory filing (investigational new drug application (IND), new drug application (NDA), or biologics license application (BLA)), but in practice, pharmaceutical companies are reluctant or do not include new excipients in their applications over concerns that inclusion of a new pharmaceutical ingredient other than the active ingredient in a drug formulation may raise additional regulatory concerns and possibly jeopardize a drug filing. Proponents of an FDA novel excipient review program say that the FDA’s recognition of a novel excipient would reassure drug developers that the novel excipient can be used in a drug-development program while minimizing the risk that safety concerns would be raised by the FDA during application review. They have also cited a perceived risk aversion on the part of drug developers, such that novel excipients may be avoided in drug-development programs, even when the excipients have potential health benefits. A novel excipient review program, therefore, would allow companies to know in advance if a new excipient was acceptable from a regulatory view without running the risk of an IND, NDA, or BLA being rejected or delayed due to questions over a novel excipient. Adjusting the regulatory review process as such, some say, would encourage the development of novel excipients, which is important to provide pharmaceutical companies and excipient manufacturing the needed flexibility to develop new excipients as may be needed to resolve drug-formulation changes.
The US Food and Drug Administration (FDA) took a first step in considering such an approach late last year (December 2019) by issuing a request for input from the industry for purposes of obtaining information and comments that will assist the agency in determining whether it should establish a pilot program for the toxicological and quality evaluation of novel excipients intended for use in human drugs. The agency was interested in obtaining information and comments on several aspects of such a program before deciding whether to develop it. The industry had until early February (February 2020).
The FDA was interested in getting input from the industry on several key questions, including would such an approach overcome drug developers’ reluctance in using novel excipients and what criteria should be required for the novel excipient (see Figure 1).
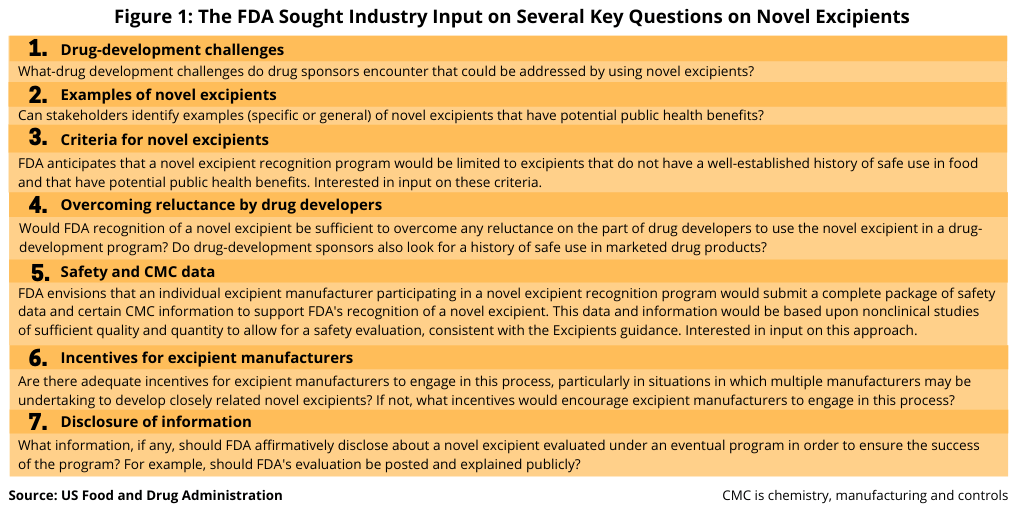
Drug-development challenges. What-drug development challenges do drug sponsors encounter that could be addressed by using novel excipients?
Examples of novel excipients. Can stakeholders identify examples (specific or general) of novel excipients that have potential public health benefits?
Criteria for novel excipients. The FDA says that it anticipates that a novel excipient recognition program would be limited to excipients that do not have a well-established history of safe use in food and that have potential public health benefits and was interested in stakeholder comment on these criteria.
Overcoming reluctance by drug developers. Would FDA recognition of a novel excipient be sufficient to overcome any reluctance on the part of drug developers to use the novel excipient in a drug-development program? Do drug-development sponsors also look for a history of safe use in marketed drug products?
Safety and CMC data. The FDA says it envisions that an individual excipient manufacturer participating in a novel excipient recognition program would submit a complete package of safety data and certain CMC (chemistry, manufacturing, and controls) information to support the FDA’s recognition of a novel excipient. This data and information would be based upon nonclinical studies of sufficient quality and quantity to allow for a safety evaluation, consistent with the FDA’s excipients guidance, Nonclinical Studies for the Safety Evaluation of Pharmaceutical Excipients. The FDA was interested in stakeholder comment on this approach.
Incentives for excipient manufacturers. Are there adequate incentives for excipient manufacturers to engage in this process, particularly in situations in which multiple manufacturers may be undertaking to develop closely related novel excipients? If not, what incentives would encourage excipient manufacturers to engage in this process?
Disclosure of information. What information, if any, should FDA affirmatively disclose about a novel excipient evaluated under an eventual program in order to ensure the success of the program? For example, should the FDA’s evaluation be posted and explained publicly?
FDA’s view of a possible novel excipient review pilot program
In seeking input on a novel excipient review program, the FDA put forth what such a pilot program would be. The pilot program would review a limited number of submissions per year. Any program developed by the agency would be voluntary, and that FDA recognition of a novel excipient would not be necessary for the novel excipient to be included in a finished drug product described in an IND, an NDA, or a BLA.
Generally, the FDA says it anticipates that a submission to a potential novel excipient review program would include toxicological studies supporting the safety of the novel excipient at anticipated levels and duration of exposure by anticipated routes of administration. Additionally, the FDA anticipates that submitters would provide identification and control information, including compositional and purity specifications for the novel excipient as outlined in FDA guidance.
The FDA’s recognition of a novel excipient would mean that, based on a review of safety, manufacturing, and compositional information, the FDA has determined that the proposed context of use (e.g., acute or chronic exposure by specified route(s) of administration up to specified amount(s)) is expected to be safe. This determination would obviate the need for FDA review of the excipient in the context of an IND if its use in the investigational product is consistent with the recognized context of use. In the case of an NDA or a BLA seeking marketing approval or licensure of a finished drug or biological product containing a recognized excipient, the FDA would review all information in the application relating to safety of the finished product. The FDA expects that excipients reviewed under this program, after they are used in approved formulations, would be listed in the FDA’s Inactive Ingredient Database, which is a listing of inactive ingredients (i.e., excipients) present in FDA-approved drug products currently marketed for human use.
Industry feedback
Companies and industry associations and organizations offering input on the FDA’s information request have been largely supportive of a novel excipient review program by the FDA.
The International Pharmaceutical Excipients Council of the Americas (IPEC-Americas), which consists of excipient manufacturers, pharmaceutical companies, CDMOs/CMOs involved in drug formulation and drug-product manufacturing, and distributors, supports a pilot program for novel excipients. “IPEC-Americas strongly supports establishing a pilot program for evaluating the toxicology and quality of novel excipients intended for use in human drugs,” said Janeen Skutnik-Wilkinson, Chair, IPEC-Americas, in comments submitted to the FDA on January 14, 2020. “Furthermore, IPEC-Americas believes that a Novel Excipient Review Program would encourage early development and use of novel excipients to facilitate advancing the next generation of medicines to patients and help stimulate excipient innovation in the global pharmaceutical industry.”
In detailed comments, IPEC-America’s Skutnik-Wilkinson, outlined benefits of such a program. “Currently, there is no system for formulators to know the status of novel excipients since the inactive ingredient database (IID) currently only lists excipients with a precedence of use. By implementing a novel excipient review program and posting information about novel excipients that have been reviewed and found to be generally acceptable for certain applications, FDA could provide opportunities for enhanced innovation.”
In addition, IPEC-Americas recommends that the FDA further define and clarify what is meant by a “novel excipient.” The organization recommended that a novel excipient review program be broadened and staged (meaning the degree of newness for different types of novel excipients will influence the amount of safety data required to complete an appropriate assessment) to include the following types of excipients: (1) co-processed and/or mixed excipients; (2) new grades within the same chemical family; (3) food and cosmetic additives used for the first time in drugs; (4) excipients used at higher levels than listed in the Inactive Ingredient Database; (5) excipients used in modified routes of delivery than those listed in the Inactive Ingredient Database; (6) chemically modified excipients; and (7) new chemical entities.
“Since the degree of newness for different types of novel excipients will influence the amount of safety data required to complete an appropriate assessment, IPEC-Americas recommends that the program follow a multi-stage approach,” said IPEC-America’s Skutnik-Wilkinson. “Although IPEC-Americas would support starting with a pilot to evaluate new chemical entities (NCE) for INDs, NDAs and BLAs, the number and type of NCEs are currently limited. IPEC-Americas believe that, additionally, one of the biggest obstacles to innovation is with non-NCE excipients that are also classified as novel excipients by FDA. The program needs to ultimately be expanded to also allow for the evaluation of these types of novel excipients for INDs, NDAs, BLAs and ANDAs [abbreviated new drug applications].”
While suggesting refinements to a novel excipient review program, overall, IPEC-Americas supports such a program to create the needed incentives for excipient manufacturers and pharmaceutical companies to develop and use novel excipients. “The current environment provides many disincentives for excipient manufacturers to develop any type of novel excipient,” said IPEC-Americas in its comments. “For instance, during early development of a novel excipient, industry has no way to know if it will be acceptable for use. Safety data in human drugs is not available prior to an NDA approval. In some cases where excipients have been previously used in foods and/or cosmetics, relevant safety data may exist. Within the current system, there is limited incentive for an excipient manufacturer to develop a novel excipient or for a formulator to evaluate it unless all other formulation approaches have been unsuccessful. A common phrase in the industry relative to the use of novel excipients is that a pharmaceutical company wants to be ‘first to be second.’ They are not willing to be the first company to use a novel excipient because of the regulatory uncertainty. The proposed Novel Excipient Review Program would be seen as a critical step towards incentivizing and sustaining investment in innovative new materials. Providing a pathway for novel excipients to be reviewed prior to their use in an approved drug product would be seen very positively by both excipient manufacturers and drug formulators.”
The Drug Product Leadership Group of the International Consortium for Innovation and Quality in Pharmaceutical Development (IQ), a technically focused organization of pharmaceutical and biotechnology companies, also supports a novel excipient review program. “The IQ Consortium strongly supports establishing a pilot program for evaluating the safety and quality of novel excipients intended for use in human drugs,” said the organization in comments submitted to the FDA on February 3, 2020. “The IQ Consortium believes that a Novel Excipient Review Program will encourage the pharmaceutical industry to develop and use novel excipients, which in turn would facilitate the development of safe, efficacious and innovative medicines to address therapeutic and patient needs.”
The United States Pharmacopeia (USP), a non-profit scientific and standards-setting organization, also supports a novel excipient review program. USP developed and launched a novel excipients survey in March 2019, the goal of which was to better understand the current drug approval pathway’s impact on novel excipient innovation and to identify challenges and the current state of innovation by stakeholders. “The survey results indicated that the current regulatory approval pathway for excipients creates a challenge for the use of novel excipients,” said USP in its comments to the FDA on January 27, 2020. “Based on the survey results, USP is supportive of a novel excipient review program since it provides a pathway for excipients outside of the normal application review process.”
USP also outlined ways in which the organization could facilitate a novel excipient review program through the United States Pharmacopeia—National Formulary (USP–NF), its compilation of quality standards. In its comments, it said it could explore modifying its pending monograph process (PMP) to clarify how the process could be utilized to facilitate revision of existing USP–NF excipient monographs and potential new monographs for excipients that are being reviewed as part of an FDA drug application. Using the PMP for the creation of an excipient monograph would provide a mechanism for determination of the appropriate identification, compositional, and purity specifications for the novel excipient that coincides with the FDA’s review and approval of the associated application.
Furthermore, USP said in its comments it is working on developing a general chapter that focuses on quality information, including chemistry, identity, and other specifications for excipients that will support industry and the FDA’s review as part of the novel excipient review program. USP said it is interested in discussing approaches with the FDA on how to establish identity specifications for novel excipients that have been evaluated by the FDA. “The availability of standardized identity information for a novel excipient could help industry and the FDA in its establishment and evaluation of quality specifications for novel excipients,” said USP in its comments.







